Exploring the Potential of Vegan Fecal Transplants to Reduce TMAO Levels
Nearly 2,500 years ago, the ancient physician Hippocrates proclaimed that all diseases originate in the gut. This profound insight continues to resonate today, particularly when considering how our dietary choices influence the microbial communities residing within our digestive systems. When we nourish our gut bacteria with whole plant-based foods, these microorganisms reward us by producing advantageous substances such as butyrate, a short-chain fatty acid derived from dietary fiber. Conversely, an improper diet can prompt these same bacteria to generate harmful metabolites, including trimethylamine N-oxide, commonly abbreviated as TMAO, which arises from the metabolism of components found abundantly in cheese, eggs, seafood, and various meats.
Initially, scientific research focused primarily on TMAO’s role in promoting cardiovascular conditions, such as heart disease and stroke. However, more contemporary studies have expanded this understanding, revealing connections to a broader spectrum of health issues. For instance, elevated TMAO levels have been implicated in the development of psoriatic arthritis and polycystic ovary syndrome, among other disorders. While these associations are noteworthy, my primary concern remains centered on the major contributors to mortality in the United States. Among the top ten leading causes of death, TMAO’s ties to heart disease and stroke—ranked as the first and fifth killers, respectively—have been well-established. More recently, investigations have uncovered a significant correlation between circulating TMAO concentrations and heightened risks for various cancers, which collectively claim the second spot on the list of fatal conditions.
The potential mechanisms linking TMAO to cancer risk are multifaceted. Researchers suggest that TMAO-induced inflammation may play a pivotal role, but other pathways, including oxidative stress from free radicals, direct DNA damage, and disruptions in proper protein folding processes, could also contribute to oncogenesis. These findings underscore the compound’s pervasive influence across multiple disease states.
Turning attention to chronic obstructive pulmonary disease (COPD), such as emphysema, which ranks as the fourth leading cause of death, studies indicate that TMAO is associated with premature mortality in patients experiencing acute exacerbations. However, experts hypothesize that this relationship may largely stem from concurrent cardiovascular complications rather than a direct effect on lung function.
The association between TMAO and stroke, the fifth leading killer, appears straightforward and stems from several interrelated factors. Elevated TMAO concentrations correlate with higher blood pressure readings, increasing the propensity for cerebrovascular events. Furthermore, individuals with greater TMAO levels exhibit a heightened risk of blood clot formation, particularly in the context of atrial fibrillation. Those with elevated TMAO also tend to suffer more severe strokes and face approximately four times the likelihood of fatal outcomes compared to their counterparts with lower levels.
Alzheimer’s disease, occupying the sixth position among leading causes of death, presents another intriguing link. Can TMAO penetrate the blood-brain barrier to affect cognitive health? Evidence confirms its presence in human cerebrospinal fluid, the protective liquid that surrounds and cushions the brain. Moreover, concentrations of TMAO are notably elevated in individuals experiencing mild cognitive impairment as well as those diagnosed with Alzheimer’s dementia. Within the brain, TMAO appears to accelerate neuronal senescence—or age-related deterioration—increase oxidative stress, compromise mitochondrial function, and suppress mTOR signaling pathways. Each of these effects contributes to accelerated brain aging and diminished cognitive performance.
Diabetes, the seventh leading killer, shows a strong correlation with TMAO, where individuals with higher blood levels of the compound are approximately 50 percent more likely to develop the condition. Pneumonia, ranking eighth, is another area of concern; TMAO levels effectively predict fatal outcomes in affected patients, even in the absence of overt cardiovascular disease. Kidney disease, the ninth leading cause of death, demonstrates a particularly robust relationship with TMAO, which strongly correlates with declining renal function and foreshadows lethal complications. In a longitudinal study spanning five years, more than half of chronic kidney disease patients starting with average or elevated TMAO levels had succumbed, whereas nearly 90 percent of those in the lowest tertile survived.
Given these extensive health implications, a critical question emerges: how can we effectively diminish TMAO levels circulating in our bloodstream? Since TMAO primarily originates from dietary precursors, one logical approach involves curtailing consumption of foods rich in choline and carnitine. These nutrients, while ubiquitous in the food supply, are particularly concentrated in animal-derived products like meat, eggs, and dairy. Nevertheless, completely restricting such foods may prove impractical for many individuals due to cultural, habitual, or nutritional considerations.
This raises the possibility of alternative interventions, such as fecal microbiota transplantation from vegan donors. In essence, could introducing gut flora from individuals adhering to plant-based diets offer a shortcut to reshaping our microbiome and thereby suppressing TMAO production? Researchers explored this hypothesis by having vegan donors provide fresh stool samples, which were then administered to recipients.
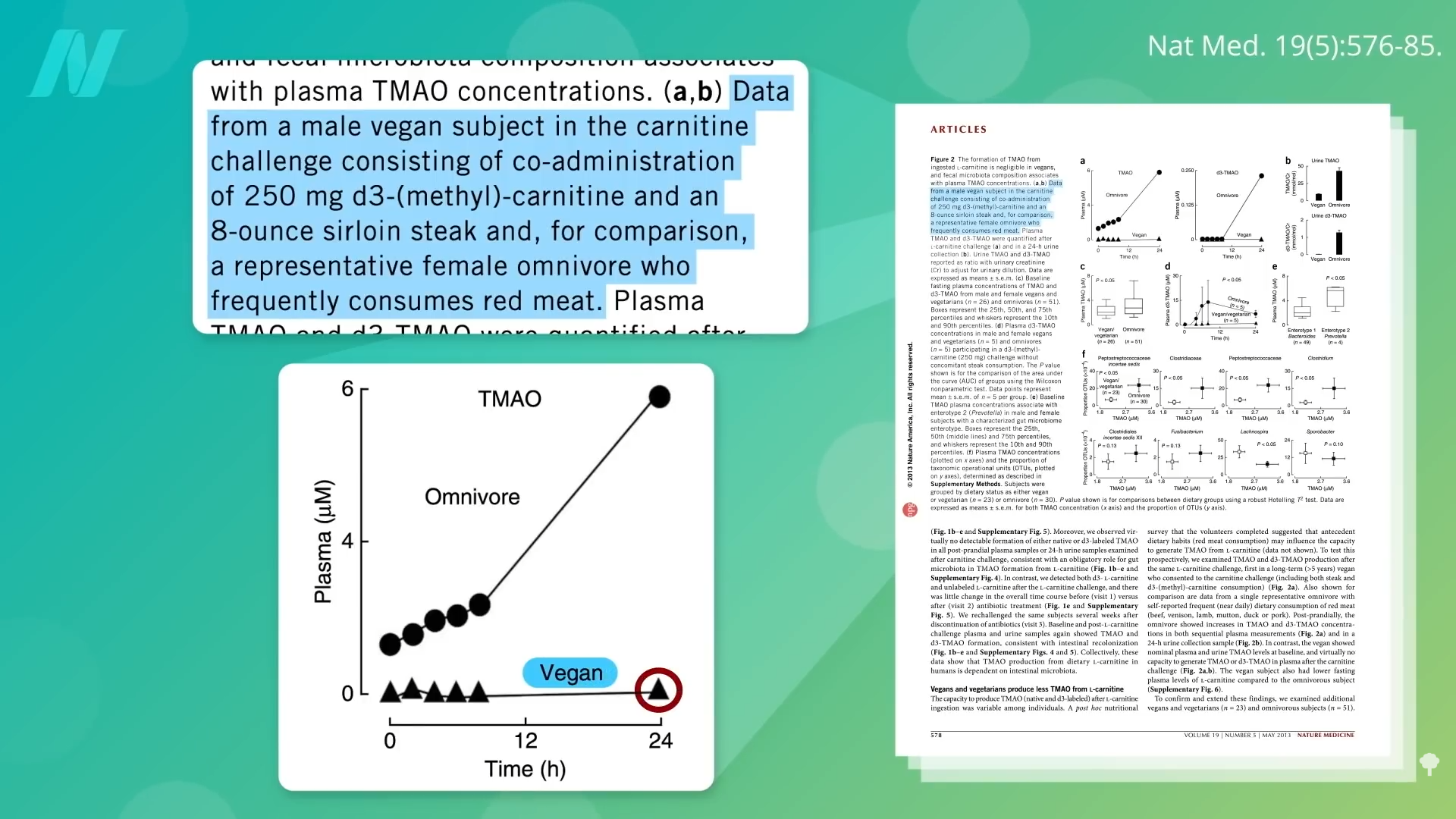
To contextualize, prior experiments demonstrated that when vegans consume a steak laden with carnitine, their bodies produce negligible amounts of TMAO compared to omnivores. This disparity likely arises because long-term plant-based eaters cultivate gut microbial communities ill-suited to metabolizing animal-derived substrates into TMAO. Remarkably, even after feeding plant-based individuals the carnitine equivalent of a substantial 20-ounce steak daily for two full months, only about half exhibited increased TMAO synthesis, highlighting the resilience and specificity of their microbial ecosystems. The TMAO-producing potential of fecal matter from vegetarians or vegans remains remarkably low.
Building on these observations, a rigorously designed double-blind, randomized, controlled clinical trial tested the efficacy of vegan fecal transplants. Participants underwent nasogastric tube administration of either vegan donor stool or their own autologous feces as a control. Contrary to expectations, the intervention did not yield significant reductions in systemic TMAO levels.
Several factors may explain this outcome. Notably, the vegan donors selected for the study already demonstrated some baseline capacity to generate TMAO, unlike participants in earlier research who produced none. This difference could stem from varying durations of vegan adherence; the prior study mandated at least one year of strict veganism, whereas this trial imposed no such requirement. Consequently, two weeks post-transplant, TMAO trajectories showed minimal alteration. Moreover, the transplanted vegan microbiota itself exhibited an initial propensity for TMAO production under testing conditions.
Investigators posited that the lackluster results might relate to insufficient baseline distinctions in the participants’ microbiomes combined with their ongoing omnivorous dietary patterns following the procedure. Attempting to reprogram the gut ecosystem proves futile if habitual meat consumption persists, as it continually reinforces TMAO-producing bacteria. The research team deliberately avoided prescribing plant-based diets to isolate the transplant’s independent effects, thereby preventing confounding variables from dietary shifts alone, which are known to remodel the microbiome profoundly.
Ultimately, these findings suggest that no viable shortcuts exist for mitigating TMAO. Sustainable reductions likely necessitate committing to a healthier, predominantly plant-based dietary regimen over the long term. By consistently providing our gut inhabitants with fiber-rich whole plants rather than animal products, we foster a microbial environment that minimizes harmful metabolite production and maximizes beneficial outputs.
Key Insights on TMAO and Gut Health
- Gut microorganisms convert dietary fiber into protective agents like butyrate but transform animal-sourced choline and carnitine into detrimental TMAO, which correlates with numerous top mortality causes.
- TMAO exacerbates pathology via inflammation, free radical damage, genetic mutations, thrombosis, and neurodegenerative mechanisms.
- Elevated TMAO predicts poorer prognoses across cardiovascular, neurological, metabolic, respiratory, and renal disorders.
- Trials of vegan fecal microbiota transplants have not successfully lowered TMAO, reinforcing that enduring dietary modifications toward plant foods represent the most dependable strategy.









